Recently in the New York Times, Carl Zimmer wrote about a new study concluding that misconduct is the primary reason for the growing number of retractions in scientific journals. That included plagiarism and other bad behavior, but fraud was suspected about 43 percent of the time.
This is hardly ever like fraud in the financial world. An exception might occur when the experiment involves, for example, a potentially lucrative cancer drug. In a piece today for Reuters, Sharon Begley describes how the results of a clinical trial may have been manipulated so that a treatment for prostate cancer sounded better than it really was. At best Provenge adds only a few months to life, but even that might have been an illusion. The placebo may have hastened the deaths of people in the control group — the ones not getting the drug.
More often, as Maggie Koerth-Baker points out, the reasons for fudging data are subtle:
It’s about having spent years on a project and really, really, really not wanting to believe that time was wasted. . . . It’s about convincing yourself that you can cheat a little, just this one time, because your particular circumstances are just.”
You know in your heart that your hypothesis is right. You rush to stake your claim, even if the data didn’t quite cooperate.
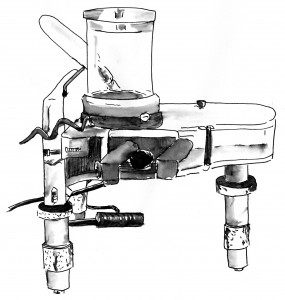
In The Ten Most Beautiful Experiments, I wrote about allegations that Robert Millikan cherry-picked his data when he became the first to measure the charge of the electron. That is more important than it might sound. Before Millikan it was unclear whether electricity came in a continuous flow like water or was parceled out in precise units, like pocket change. It is a difficult experiment, and I describe how I tried it at home with an old Millikan apparatus from eBay and a 10,000-volt power supply.
The idea is to use a perfume atomizer to spray a cloud of oil droplets into a gap between two brass plates and then apply a high voltage, manipulating the dial until a droplet is suspended in mid air. Then you let go, timing its fall with a stopwatch. After you have followed a dozen or so, you plug the numbers into some equations and calculate the fundamental unit of charge.
It’s scary work and I failed miserably. If science had depended on me, electrons would come in all shapes and sizes. Or there would be no electrons.
These things sound so easy in the physics books. You don’t hear about the brass plates shorting out and sparking because a metal clip slipped into the wrong position. . . . I’d confuse one drop with another or with a floater in my eye. . . . Sometimes a drop would be so heavy that it sank like a stone, or carry so much charge that when I turned on the voltage it rocketed out of sight. I tried and failed too many times before I realized: for me to master so delicate an experiment would be like learning to play the violin or at least make good cabinetry.”
Maestro Millikan, I called him.
Here are some observations from his notebooks:
Very low something wrong . . . not sure of distance . . . Possibly a double drop . . . Beauty Publish . . . Good one for very small one . . . Exactly Right . . . Something the matter . . . Will not work out . . . Publish this Beautiful one. . . . Perfect Publish . . . Best one yet. . . .
Years after Millikan’s death, entries like these led to suspicions that he had cooked the books. I found myself siding with his defenders.
This is not an accusation that rings true to someone who has struggled with the oil-drop experiment. Millikan, I suspect, had simply developed a feeling for the mechanism, a sixth sense for when something had gone wrong: a slip of the thumb on the stop watch, a sudden fluctuation in temperature or plate voltage, a dust particle masquerading as an oil drop. He knew when he had a bad run.
More interesting than the unfounded allegations is the question of how you keep from confusing your instincts with your suppositions, unconsciously nudging the apparatus, like a Ouija board, to come up with the hoped-for reply. It’s something every experimenter must struggle with. The most temperamental piece of laboratory equipment will always be the human brain.”
The charge, Millikan concluded, was 1.5924 x 10-19 coulombs. (One coulomb is about what flows each second through a 100-watt bulb.) A century later the accepted value is 1.6022 x 10-19.
Follow @byGeorgeJohnson